Analytical and quality control
Product characterization
Demonstration of biosimilarity relies on comprehensive comparability studies with the reference drug. Parameters such as the tertiary structure of proteins, post-translational modifications, such as glycosylation profiles, charge variants, and other product quality characteristics can vary widely with what are considered minor changes in the product manufacturing process. Therefore, the safety and effectiveness features of these products are greatly affected. Control concepts and quality issues are related to the entire production process. In cooperation with famous national and international companies, we provide a comprehensive biopharmaceutical analysis and design and implement fast and accurate analytical tests to study the biosimilarity of the product.
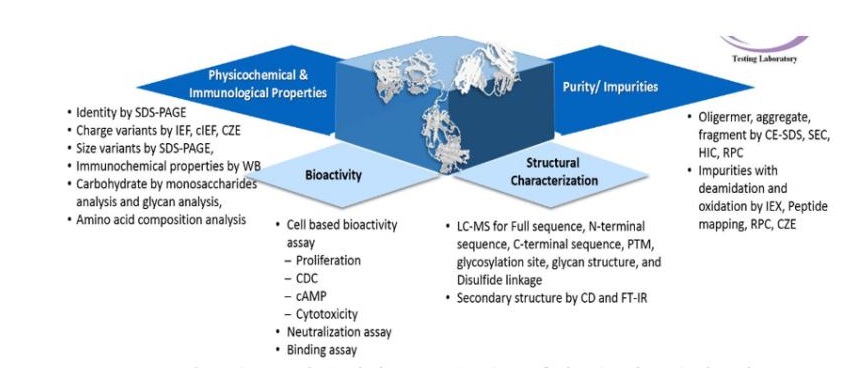
Comprehensive analytical characterization of physicochemical and immunological properties
Establishment of quality target products profile (QTPP) based on quality by design principles and scientific knowledge
Ensured consistency of product quality through characterization or comparative analysis during process development
Comprehensive analytical characterization of reference products and biosimilarity assessment
